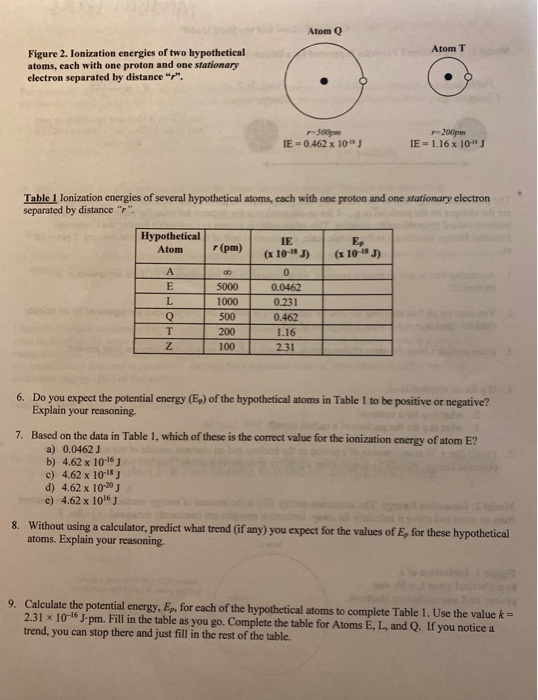
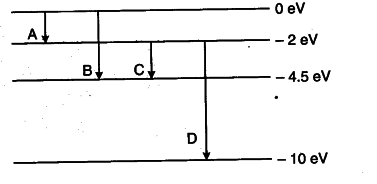
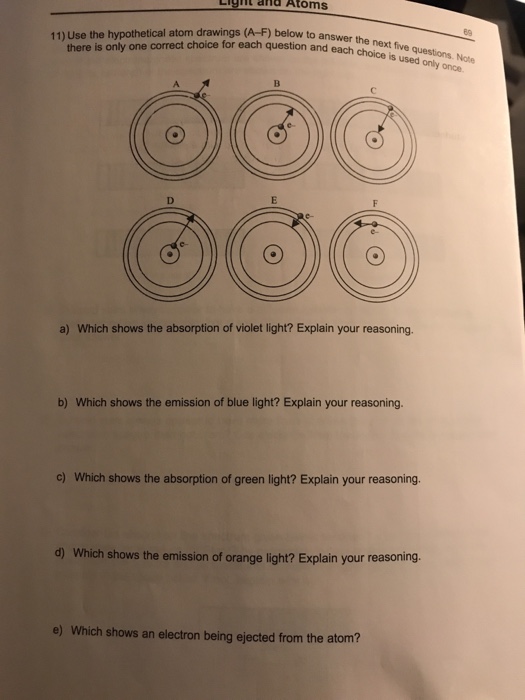
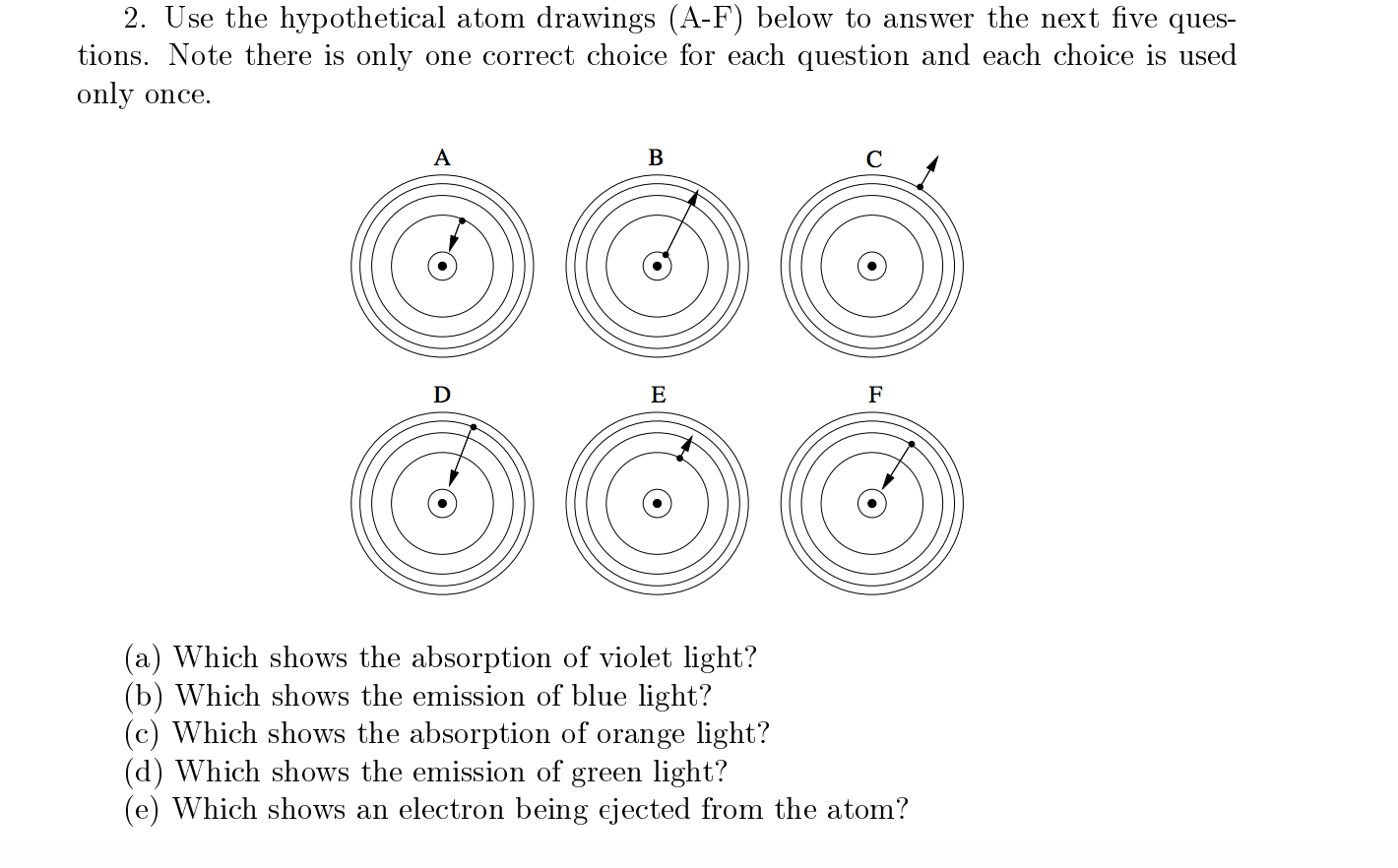
The energy levels of an atom are shown below. Which of them will result in the transition of a photon of wavelength 275 nm?

Suppose that a hypothetical atom gives a red, green, blue and violet line spectrum. Which jump according to figure would give off the red spectral lin - Sarthaks eConnect | Largest Online

Schematic energy levels of a hypothetical atom with configurations d 2... | Download Scientific Diagram

Schematic energy levels of a hypothetical atom with configurations d 2... | Download Scientific Diagram
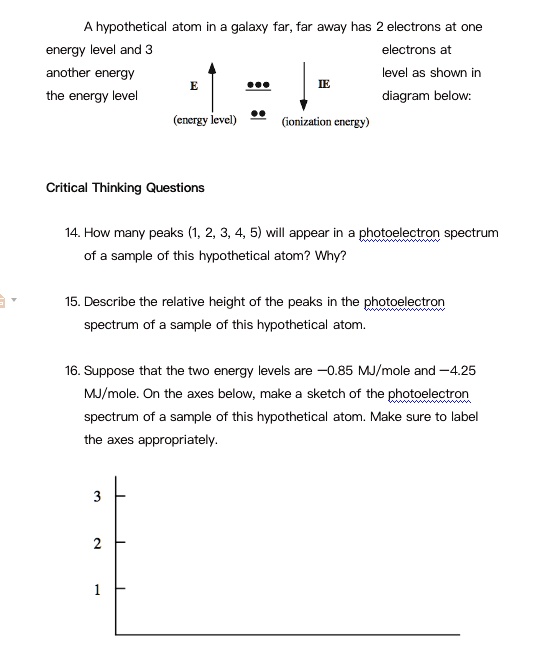
SOLVED: hypothetical atom in galaxy far, far away has electrons at One energy level and 3 electrons at anotner energy the energy level level as shown in diagram below: (ercizy levcl) (ionization

The energy levels of a hypothetical atom are given below. Which of the shown transitions will result in the emission of photon of wavelength 275 nm - Gurukul For JEE & NEET
The energy level diagram is for a hypothetical atom. A gas of these atoms initially in the ground - Sarthaks eConnect | Largest Online Education Community

The energy levels of a hypothetical atom are shown below. Which of the shown transitions will result in the emission of photon of wavelength 275nm?

Suppose that a hypothetical atom gives a red, green, blue and violet line spectrum. Which jump - YouTube

The energy levels of a hypothetical atom are given below. Which of the shown transitions will result in the emission of photon of wavelength 275 nm - Gurukul For JEE & NEET
An energy-level diagram for a hypothetical atom is shown above. - Sarthaks eConnect | Largest Online Education Community

4: Two-level energy diagram of a hypothetical atom in the presence of a... | Download Scientific Diagram

The energy levels of an atom are shown below. Which of them will result in the transition of a photon of wavelength 275 nm?

In a hypothetical model of an atoms following Bohr's theory, the potential energy is given by potential energy =-(Ke^(2))/(4r^(4)). Which of the following options will be correct ? (Symbols have usual meaning)
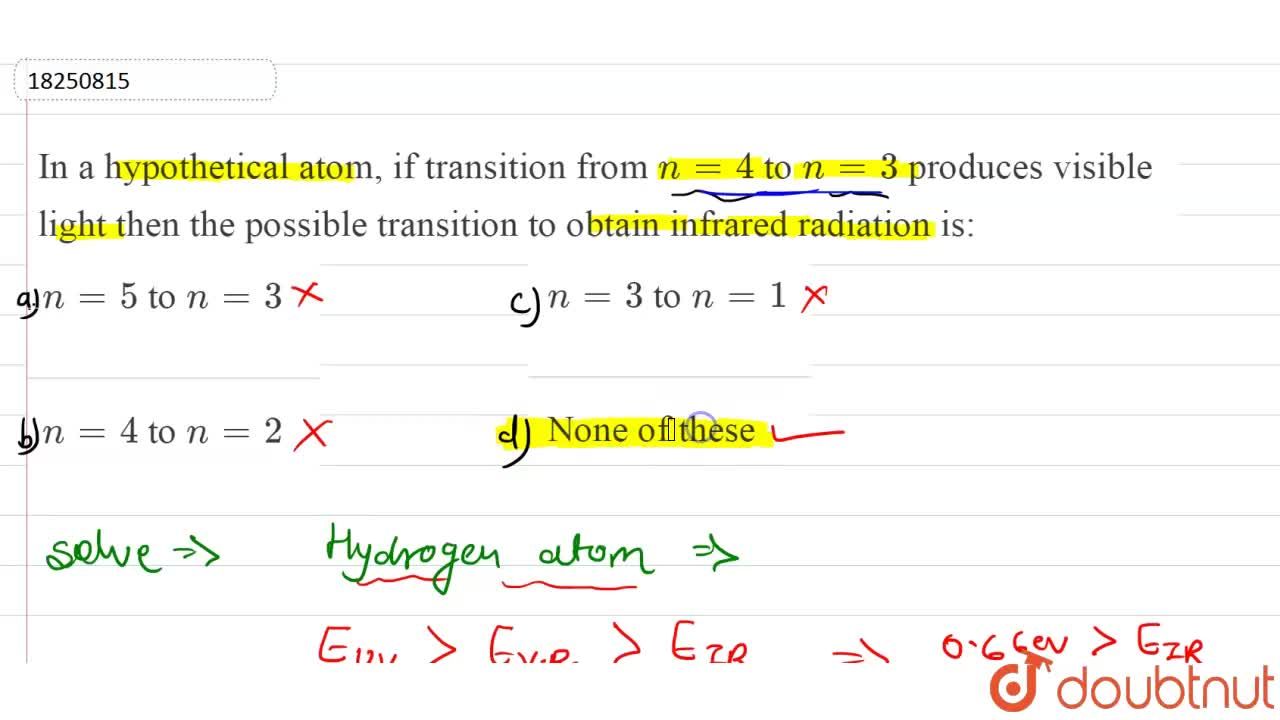
In a hypothetical atom, if transition from n=4 to n=3 produces visible light then the possible transition to obtain infrared radiation is:


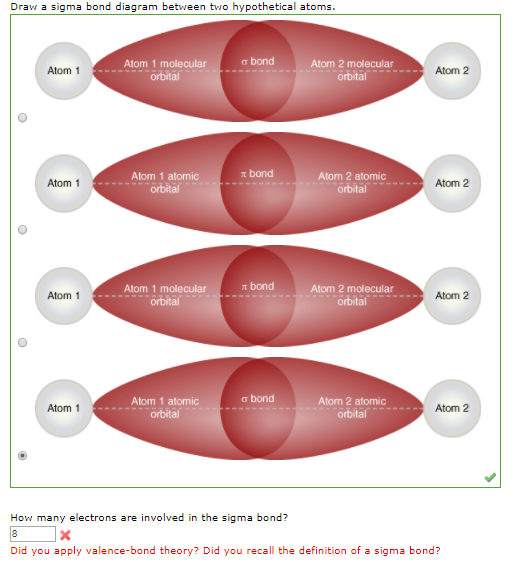
![ANSWERED] Consider the hypothetical atom "X". If the... - Organic Chemistry ANSWERED] Consider the hypothetical atom "X". If the... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/45022300-1658615686.1122277.jpeg)